Abstract
Background
Anemia in inflammatory bowel disease (IBD) presents as a common extraintestinal manifestation resulting in many complications. This condition is often missed or underrated, anemia is secondary to blood loss or defective absorption of iron which can result in a combination of iron deficiency anemia (IDA) or anemia of chronic disease (ACD). The management is iron replacement therapy which improves the quality of life in these patients. Due to the constraints in the use of oral iron, parenteral preparations are more used in IBD patients. Commonly used iron sucrose and ferric carboxymaltose are often associated with side effects leading to poor compliance. Our study explores data about ferric derisomaltose also known as iron isomaltoside (IIM), a recently approved IV iron preparation. The FDA approved this drug in 2020 for patients with poor compliance with other iron preparations. We explored the efficacy and safety data of ferric derisomaltose in adult patients with IBD.
Material/Methods
A literature search was performed using the following databases: PubMed, Cochrane, Embase, Clinical trials.gov, and Web of Science. The search was completed without using any filter and we used the MeSH Terms for "anemia", "iron deficiency anemia", "inflammatory bowel disease", and "ferric compounds". A total of 1590 articles were screened, and we finally selected 2 trials and 2 observational studies. We followed the PRISMA guidelines for literature search and selection of studies. Case reports, preclinical trials, meta-analyses, and review articles were excluded. Trial and observational studies related to IBD were included.
Results
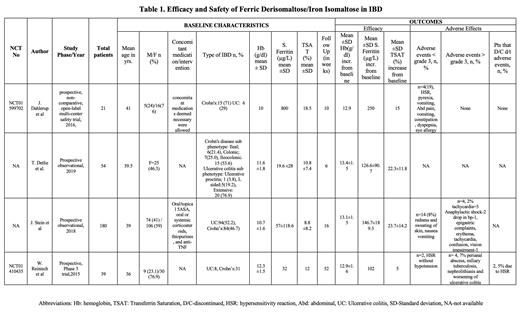
In total, 294 patients with anemia in inflammatory bowel disease received ferric derisomaltose intravenously as a single dose between 500 mg to 2000 mg. All patients were >18 years of age. The mean pre-treatment hemoglobin (Hb) level varied from 10.0 g/dL to 12.3 g/dL. The mean serum ferritin ranged from 19.6 µg/L to 57 µg/L and the mean transferrin saturation (TSAT) ranged from 8.8% to 18.5%. J. Stein et al investigated the effectiveness and safety of IIM in routine practical care of IDA in IBD patients. Dahlerup et al (NCT01599702) compared single dose IIM with multiple dosages of IIM in IBD patients. The remaining two studies investigated the effectiveness and safety of high dose IIM in patients with IBD. Results are summarized in Table 1.
Efficacy:
The increase in hemoglobin ranged from 0.6 g/dl to 2.9 g/dl from baseline while the increase in serum ferritin ranged between 102 µg /L to 250 µg /L and transferrin saturation increased in the range of 15.0%-23.7% in 10 to 52 weeks post iron isomaltoside (IIM) therapy. All markers of iron deficiency anemia showed significant improvement. In the observational study by J. Stein et al, the hemoglobin increased from a mean of 10.7 g/dl to 13.1 g/dl, serum ferritin increased from 57 µg /L to 146 µg /L, and TSAT from 8.8% to 23.7% at 16 weeks. Dahlerup et al demonstrated a Hb increase of >2 g/dl in 75% of patients at 10 weeks. According to W. Reinisch et al (NCT 01410435), there was a mean increase in serum ferritin from 32 µg /L to 102 µg /L and a decrease in TSAT from the baseline, but this decrease was not statistically significant.
Safety:
Adverse events were noted in 8 (3.6%) patients. Out of the 8 patients, 4 (<2%) were serious adverse events such as perianal abscess, miliary tuberculosis, nephrolithiasis, and worsening of ulcerative colitis, which may not be related to the study drug. The other 4 (1.8%) had life-threatening events, anaphylaxis being the most common. All four patients recovered. The most common mild adverse events reported were hypersensitivity reaction (HSR) seen in 3 patients. 2 patients discontinued due to mild HSR. There were no reports of death due to side effects of the drug.
Conclusion
Ferric derisomaltose has demonstrated a substantial increment in iron parameters in anemia in patients with IBD. This was measured in terms of hemoglobin response, serum ferritin, and transferrin saturation. Greater Hb response was achieved irrespective of concomitant treatment with steroids and anti-TGF-ß.The adverse events were mild, and patients recovered showing high compliance. Since the average duration of follow-up in our study was 21 weeks, long-term follow-up is limited for this drug, we need further studies to assess the need for maintenance therapy.
Anwer: GlaxoSmithKline: Research Funding; BMS / Celgene: Honoraria, Research Funding; Janssen pharmaceutical: Honoraria, Research Funding; Allogene Therapeutics: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal